MAKING OF SODIUM CARBONATE BY SOLVARY PROCESS
Sodium carbonate is known as washing soda or soda ash. It is commonly occurred as a crystalline heptahydrate which readily effloresces to form a white powder, the monohydrate.
Pure sodium carbonate is a white, odorless powder that is hygroscopic means it absorbs moisture from air. It has an alkaline taste and forms a strongly alkaline water solution. It is produced by solvary process by using common salt (sodium chloride), ammonia and limestone.
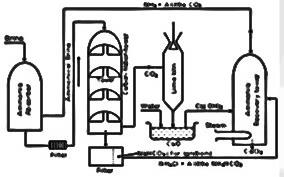
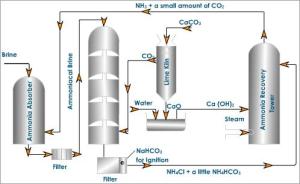
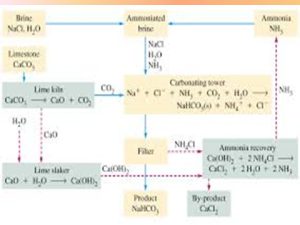
The solvary process centered on a large hollow tower. At the bottom limestone was heated to release carbon dioxide.
The steps in solvary process:
- Brine purification:
Brine is concentrated by evaporation. Impurities such as calcium, magnesium and iron are removed by precipitation.
For example:
Ca+2(aq) + CO3-2(aq) ——–à CaCO3(s)
Mg+2(aq) + 2OH–(aq) ——àmg(OH)2(s)
Fe+3(aq) + 3OH–(aq) ——àFe(OH)3(s)
After removing the impurities Brine solution is then filtered and passed through an ammonium tower to dissolve ammonia.
- Important point to remember:
It is an exothermic process, so the ammonia is cooled.
- Formation of sodium bicarbonate:
Carbon dioxide is produced by the thermal decomposition of limestone.
CaCO3——-à CO2+CaO
Carbon dioxide is bubbled through the ammoniated Brine Solution in the carbonating tower.
The carbon dioxide dissolves to form a weak acid:
CO2(g)+H2O(l)↔HCO3(aq)+H+(aq)
The ammonia in the brine reacts with H+ to form Ammonium ion.
NH3+H+↔NH4+
The bicarbonate ion reacts with Na+ to form sodium Hydrogen carbonate (sodium bicarbonate).
Na++HCO3–↔NaHCO3(s)
Sodium bicarbonate precipitates
NH3(aq)+CO2+NaCl+H2O↔NaHCO3+NH4Cl
- Formation of sodium carbonate:
The suspended sodium hydrogen carbonate is removed from the carbonating tower and heated at 300°C to produce sodium carbonate.
2NaHCO3——–à Na2CO3+CO2+H2O
This CO2 is recycled back into the carbonating tower.
- Ammonia recovery:
Calcium oxide which was formed as a byproduct of the thermal decomposition of limestone in the lime kiln, react with water to form calcium hydroxide
CaO(s)+ H2O(l)——–à> Ca(OH)2 (aq)
This calcium hydroxide reacts with ammonium chloride separated out of the carbonating tower by filtration.
Ca(OH)2+2NH4Cl—-à CaCl2+2H2O+ 2NH3
The ammonia is recycled back into the process to form ammoniated brime.
Calcium chloride is formed as a by-product of the solvey process.
Properties of sodium carbonate:
- At room temperature, sodium carbonate (Na2CO3) is an odorless, grayish white powder which is hygroscopic. This means when it is exposed to air, it can spontaneously absorb water molecules.
- Sodium carbonate has a melting point of 1,564°F (851°C), a density of 2.53 g/cm3, and is soluble in water.
- Anhydrous (without water) sodium carbonate can absorb various amounts of water and form hydrates which have slightly different characteristics.
Uses of sodium carbonate:
- It is use for glass making.
- It is used for paper making.
- It is used for production of baking soda.
- It acts as water softening agent.
This is extremely well explanation about the preparation of sodium carbonate
LikeLike
thanks Daud Khan
LikeLike
Thanks Daud Khan, I am very please to received your well explanation about the prepartion of soudium carbonate our company is keen to venture into this project also into minierial fertilizer we seek your advice to assist us with the project our main line of business in palm oil plantation and mining we look forward to proposal and advice for the above project please forward me you phone number for me to call you my mobile phone number 60 12-8028705 look forward to your reply soon wassalam hakim khan
LikeLike
Hello Hakim khan thanks for praising the explanation
i am only a science teacher and my mission is make science topics easier for students
i am satisfied with my job
LikeLike
thank u so much
LikeLike
Thank you Dikxya thapa
LikeLike
Wonderful article.
Thanks for sharing
LikeLike
Thanks for liking the article
LikeLike
Why can’t you help me undstnd this better
LikeLike
let me know your question regarding this topic i will try to do my best
LikeLike